Introduction
Omega-3 fatty acids (O3FA) have been evaluated in various clinical settings for sickle cell disease (SCD) patients with promising results. This study aims to assess the efficacy and safety of O3FA supplements in preventing SCD complications.
Methods
We systematically searched Embase, PubMed, and Cochrane Register of Controlled Trials from inception to May 26 th, 2023. Studies that met the following criteria were included: (1) randomized controlled trials (RCTs) or nonrandomized studies of interventions; (2) comparing O3FA supplements to the standard of care (with or without hydroxyurea treatment); (3) patients with sickle cell disease; (4) and reporting at least one clinical outcomes of interest. We excluded studies (1) with unstable treatment history 3-6 months before the beginning of the studies; or (2) with patients on regular blood transfusion. Our outcomes of interest included (1) the frequency of painful vaso-occlusive crisis or acute chest syndrome requiring hospitalization; and (2) hemoglobin level after intervention. We used Review manager software to perform statistical analyses. The random effect model was applied to all analyses due to anticipated heterogeneity. Pooled mean differences and 95% confidence intervals (CI) were used to assess outcomes.
Results
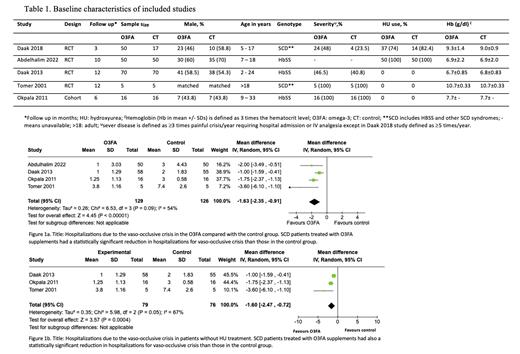
We screened 188 studies and included four RCTs and one self-controlled cohort in the meta-analysis. Our population included 349 patients aged between 2 years to adults; 54.7% received O3FA supplements. The docosahexaenoic acid (DHA) dose ranged from 10 to 60 mg per kilogram daily. Follow-up varied from 3 to 12 months. More than two-thirds were hemoglobin SS disease, as presented in Table 1. Other SCD syndromes included hemoglobin S-beta 0 thalassemia and hemoglobin SC disease. Severe baseline disease (³ 3 episodes of painful vaso-occlusive crisis per year requiring hospital admission) ranged between 23.5% and all patients among studies. The vaso-occlusive crisis episodes were significantly reduced in the O3FA group compared with standard treatment: mean difference (MD)-1.63 times (95% CI: -2.35 to -0.91; p = 0.00001; I 2 = 54%), as seen in Figure 1a. There was no difference in hemoglobin levels after intervention with O3FA versus standard treatment (MD: 0.03; 95% CI: -0.25 to 0.32; p = 0.82). In the subgroup of patients without hydroxyurea treatment, the mean frequency of vaso-occlusive crisis was significantly lower with O3FA supplements compared with standard treatment (MD: -1.60; 95% CI: -2.47 to -0.72; p = 0.0004; I 2 = 67%), as shown in Figure 1b. There were no significant adverse events (AE) in the O3FA group. The most common AE were increased appetite, dyspepsia, and occasional “fishy” smell. One patient discontinued O3FA supplements because of drug-related abdominal pain.
Conclusion
Our systematic review and meta-analysis support O3FA supplementation as a safe and effective complementary strategy to reduce painful vaso-occlusive crises in patients with SCD.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal